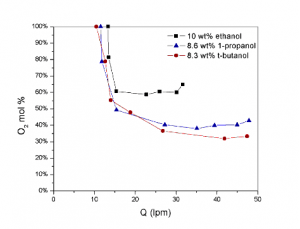
1-propanol and 8.3 wt% t-butanol solutions
Biomass is an attractive source of energy: It’s renewable and has low net greenhouse emissions, because biomass growth removes CO2 from the atmosphere. However, biomass, in its raw and processed states, generally contains a considerable amount of water. For example, the broth, in the production of bioethanol from corn, contains more than 90 wt% water. Traditionally, costly and energy-intensive dewatering processes are required to produce energy from the raw material. However, it is possible to combust biomass before removing all of the water, and LACER researchers are investigating the feasibility of this.
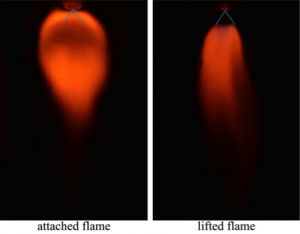
Flame stability and flame structure are being characterized for fuels that have a high water content, and the influence of preferential vaporization on flame stability is being investigated. Various water-soluble fuels are analyzed in order to identify fuels that show strong preferential vaporization. Tests for flame stability — characterized by the blow-off limit — are run for different aqueous solutions under identical flow conditions and energy content.
Ethanol, 1-propanol and t-butanol have been identified as fuels that have excellent physical and chemical properties. All burn very well, even when heavily diluted with water. The fuels are highly volatile and show strong preferential vaporization over water during droplet vaporization.
Glycerol also is being studied, as it represents a fuel with low volatility relative to water. To obtain a stable flame for low-glycerol concentrations, t-butanol or ethanol can be used as additives. Experimental results show that an attached flame can be obtained by burning a mixture of water and 8.3% t-butanol/30% glycerol or 10% ethanol/30% glycerol under oxy-fired conditions.
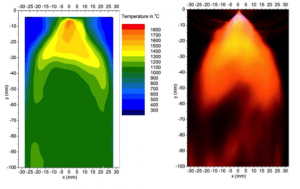
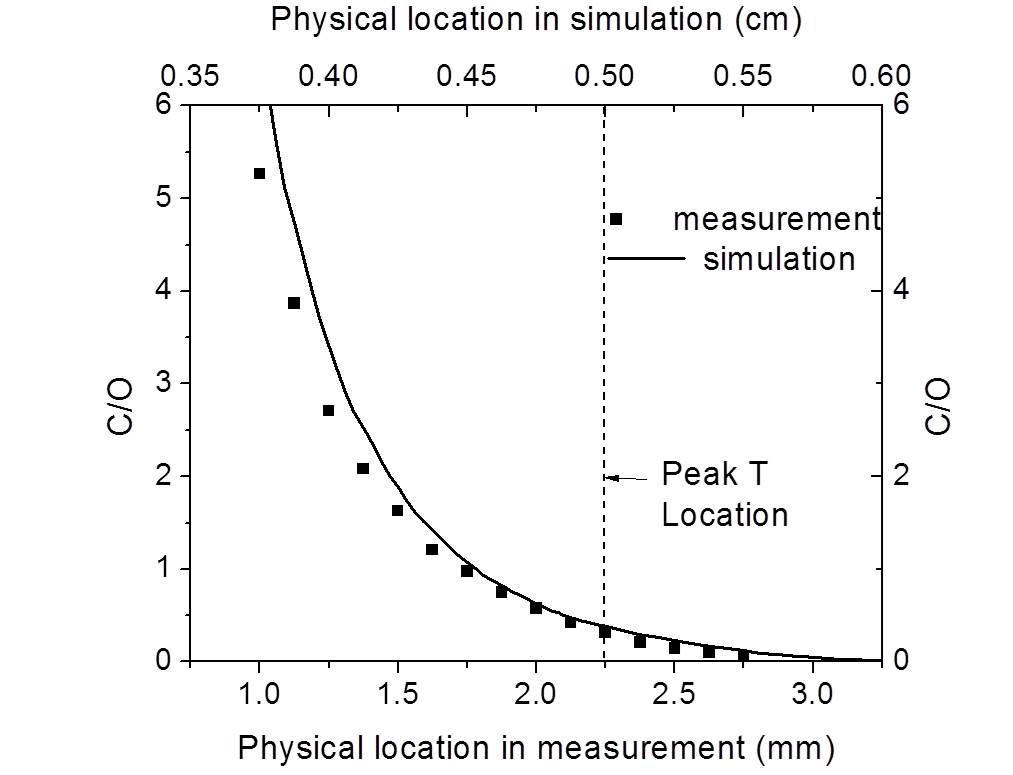
Current research involves understanding the physicochemical characteristics of fuel-water mixtures and the dynamics of their combustion. Experiments involve laser diagnostics, including Phase Doppler Particle Analyzer (PDPA) techniques for droplet size and velocity, Planar Image Velocimetry (PIV) for gas velocity, and Laser Induced Breakdown Spectroscopy (LIBS) for elemental composition.